WEAKNESS
REFLEXES
SENSATION
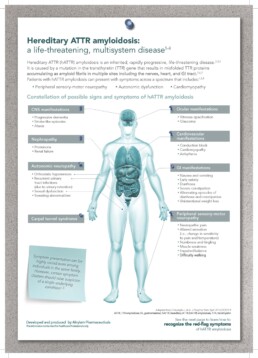
Because ATTRv amyloidosis is a systemic disease, physicians should be aware of manifestations other than those of the peripheral nervous system, such as cardiac, ocular, and renal manifestations. A multidisciplinary approach is required to assess whether, through effects of autonomic dysfunction or amyloid deposition, other organs and systems are likely to be affected 1,2,3
Autonomic dysfunction
Autonomic dysfunction occurs in approximately 73% of patients with ATTR amyloidosis with PN and affects the gut, bladder sphincter, genital nerves, and cardiovascular system.1
Evaluation of the spread of the disease
Assessment of the spread of the disease is crucial for the detection of accompanying organ damage and requires a multidisciplinary approach.1 This is essential because the involvement of most organs, other than the nervous system, is latent but may have potentially major consequences—heart blocks, restrictive cardiomyopathy, glaucoma, renal insufficiency— for patients.1
Grading of the disease
Grading of the disease in each organ system involved is important for the follow-up of these patients. Grading allows detection of eventual disease progression and of organ complications that will require specific management (Table 1). The frequency of examinations should be determined by the severity and the systemic nature of the disease in each patient.1
Follow‑up
Patients with confirmed diagnoses should be routinely followed up to monitor for disease progression .1 Assessments should evaluate somatic neuropathy with locomotion (polyneuropathy disability score), severity of sensory motor neuropathy (NIS), autonomic dysfunction, manifestations with cardiac insufficiency (NYHA), biomarkers (ECG, ECHO, NT-proBNP), BMI, renal dysfunction with eGFR, and proteinuria (Table 1).1
Desliza a derecha e izquierda para ver la tabla completa ⇆
| Evaluation | Purpose | References | |
| Neurologic manifestations A. Sensory motor neuropathy | Questionnaire | [4] | |
| Paresthesia, neurogenic pain | Small fiber loss | ||
| Gait disability | Large fiber loss | ||
| NIS (0–244) Weakness in LL and UL | Large fiber loss | ||
| Sensory loss in toes and fingers | Small and large fiber loss | ||
| Tendon reflex loss in the four limbs | Large fiber loss | ||
| Examination Pain and thermal sensory loss in the extremities in LL and UL (extension) | Small fiber loss | ||
| Disability | Modified Norris test | Sensorimotor neuropathy | [5] |
| FAP-RODS RODS | Overall disability | [6] [7] | |
| Locomotion | PND score | Autonomy to walk | |
| B. Autonomic neuropathy | CADT* (24-0) | Overall dysfunction | [8] |
| COMPASS 31 | [9] | ||
| Sudoscan | Denervated sweat glands of the soles and palms | ||
| Orthostatic hypotension | [10] | ||
| MIBG scintigraphy | Sympathetic cardiac denervation | ||
| Heart rate variability tests | Sympathetic and parasympathetic | ||
| Non-neurologic manifestations C. Cardiac | ECG, Holter-ECG Cardiac staging | Looking for conduction block or arrhythmia | |
| ECHO (strain) | Cardiac involvement | ||
| Cardiac MRI | Cardiac involvement | ||
| DPD, PYP, and HMDP scintigraphy | Cardiac amyloidosis | ||
| NT-proBNP | Cardiomyocyte stress | ||
| Cardiac troponin | Cardiomyocyte death | ||
| NYHA class | Extent of heart failure | ||
| NYHA class | Stage the extent of cardiac damage | [11] | |
| D. Ocular | Slit-lamp examination Intraocular pressure Schirmer test Visual acuity | Vitreous opacities Ocular hypertension Dry eye (sicca syndrome) | |
| E. Kidney | Proteinuria eGFR | Renal dysfunction Renal insufficiency | |
| F. General condition | Weight mBMI | Nutritional status | |
| Quality of life | Norfolk QOL-DN | Disease-specific changes in QOL | [12] |
| SF-36 QOL | Non-disease-specific changes in QOL | [13] | |
| Overall scale for ATTR disease | Kumamoto neurologic scale | Sensory disturbances, motor weakness, autonomic dysfunction, and visceral organ impairment | [14, 15] |
| Sensory motor deficit in the limbs and autonomic dysfunction | NIS + 7, mNIS + 7 | Composite score for clinical trial only | [16] [17] |
Ref. Table 5 from Adams D. (2020) Evaluation of disease progression at initial screening and follow-up
CADT Compound Autonomic Dysfunction Test, COMPASS Composite Autonomic Symptom Score, DN diabetic neuropathy, DPD diphosphono-1,2-propanodicarboxylic acid, ECG electrocardiography, ECHO echocardiography, eGFR estimated glomerular fltration rate, FAP-RODS Familial Amyloid Polyneuropathy-Specifc Rasch-built Overall Disability Scale, HMDP hydroxymethylene diphosphonate, LL lower limb, mBMI modifed body mass index, MIBG metaiodobenzylguanidine, MRI magnetic resonance imaging, mNIS modifed Neuropathy Impairment Score, NIS Neuropathy Impairment Score, NT-proBNP N-terminal fragment of the probrain natriuretic peptide, NYHA New York Heart Association, PND polyneuropathy disability, PYP pyrophosphate, QOL quality of life, SF-36 36-Item Short Form Survey, UL upper limb
- Adams D (2020) Expert consensus recommendations to improve diagnosis of ATTR amyloidosis with polyneuropathy Journal of Neurology
- Adams D (2013) Recent advances in the treatment of familial amyloid polyneuropathy. Ther Adv Neurol Disord 6:129–139
- Conceicao I, Gonzalez-Duarte A, Obici L, Schmidt HH, Simoneau D, Ong ML, Amass L (2016) “Red-flag” symptom clusters in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst 21:5–9
- Dyck PJ, Davies JL, Litchy WJ, O’Brien PC (1997) Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology 49:229–239
- Denier C, Ducot B, Husson H, Lozeron P, Adams D, Meyer L, Said G, Planté-Bordeneuve V (2007) A brief compound test for assessment of autonomic and sensory-motor dysfunction in familial amyloid polyneuropathy. J Neurol 254:1684–1688
- Pruppers MHJ, Merkies ISJ, Faber CG, Da Silva AM, Costa V, Coelho T (2015) The Val30Met familial amyloid polyneuropathy specific Rasch-built overall disability scale (FAP-RODS©). J Peripher Nerv Syst 20:319–327
- Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W (2012) COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc 87:1196–1201
- Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez-Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez-Lopez E, Lane T, Gilbertson JA, Rowczenio D, Petrie A, Hawkins PN (2018) A new staging system for cardiac transthyretin amyloidosis. Eur Heart J 39:2799–2806
- Vinik EJ, Vinik AI, Paulson JF, Merkies IS, Packman J, Grogan DR, Coelho T (2014) Norfolk QOL-DN: validation of a patient reported outcome measure in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst 19:104–114
- Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483
- Vita GL, Stancanelli C, Gentile L, Barcellona C, Russo M, Bella GD, Vita G, Mazzeo A (2018) 6MWT performance correlates with peripheral neuropathy but not with cardiac involvement in patients with hereditary transthyretin amyloidosis (hATTR). Neuromuscul Disord 29(3):213–220
- Tashima K, Ando Y, Terazaki H, Yoshimatsu S-i, Suhr OB, Obayashi K, Yamashita T, Ando E, Uchino M, Ando M (1999) Outcome of liver transplantation for transthyretin amyloidosis: follow-up of Japanese familial amyloidotic polyneuropathy patients. J Neurol Sci 171:19–23
- Dyck PJ, Kincaid JC, Dyck PJB, Chaudhry V, Goyal NA, Alves C, Salhi H, Wiesman JF, Labeyrie C, Robinson-Papp J, Cardoso M, Laura M, Ruzhansky K, Cortese A, Brannagan TH 3rd, Khoury J, Khella S, Waddington-Cruz M, Ferreira J, Wang AK, Pinto MV, Ayache SS, Benson MD, Berk JL, Coelho T, Polydefkis M, Gorevic P, Adams DH, Plante-Bordeneuve V, Whelan C, Merlini G, Heitner S, Drachman BM, Conceicao I, Klein CJ, Gertz MA, Ackermann EJ, Hughes SG, Mauermann ML, Bergemann R, Lodermeier KA, Davies JL, Carter RE, Litchy WJ (2017) Assessing mNIS+7Ionis and international neurologists’ proficiency in a familial amyloidotic polyneuropathy trial. Muscle Nerve 56:901–911
- Adams D, Cauquil C, Labeyrie C (2017) Familial amyloid polyneuropathy. Curr Opin Neurol 30:481–489
- van Nes SI, Vanhoutte EK, van Doorn PA, Hermans M, Bakkers M, Kuitwaard K, Faber CG, Merkies IS (2011) Rasch-built Overall Disability Scale (R-ODS) for immune-mediated peripheral neuropathies. Neurology 76:337–345
- Piekarski E, Chequer R, Algalarrondo V, Eliahou L, Mahida B, Vigne J, Adams D, Slama MS, Le Guludec D, Rouzet F (2018) Cardiac denervation evidenced by MIBG occurs earlier than amyloid deposits detection by diphosphonate scintigraphy in TTR mutation carriers. Eur J Nucl Med Mol Imaging 45:1108–1118